Beth Schafer lay in a hospital bed, waiting for her son to be born. The first contractions came before she felt ready, and she sensed with a mother’s deep instinct that her baby wasn’t ready either.
At just 23 weeks, her son was on the edge of viability—the fragile point where modern medicine might keep a baby alive.
When he was born, small enough to fit in one hand, he didn’t cry. A team in blue scrubs rushed to resuscitate him, trying to fill his tiny, underdeveloped lungs with air. But despite their efforts, they couldn’t give him what he needed most: more time in the womb.
Beth is a 39-year-old painter turned graphic designer. With her round glasses and tousled dark bangs, she looks like an art student who never left the studio. She’s the kind of person who offers you tea before you ask, and when she says, “I love that for you,” she means every word. That’s why I know she isn’t exaggerating when she talks about her perfect, precious son.
“I would have moved mountains for him,” Beth told me, two years after his birth.
Around the world, scientists are working to buy more time for extremely premature babies like Beth’s. In 2017, researchers in Philadelphia introduced an experimental artificial womb designed to support gestation outside the body.
In photos from their study, fetal lambs floated peacefully inside what looked like oversized plastic bags, their eyes closed and hearts beating as if they were still inside their mothers. Though the device has only been tested on animals, it’s getting closer to human trials.
In September 2023, the U.S. Food and Drug Administration met with an advisory committee to discuss whether to approve the first human trials. If approved, the first participants would be babies born between 22 and 24 weeks—less than two-thirds of the way to full term. (The FDA declined to comment on when or if these trials might start.)
In the U.S., more than 10,000 infants are born this early each year. Premature birth is the second-leading cause of infant death in the country, and those who survive often face serious complications, from chronic lung disease to lifelong neurological issues.
Artificial wombs could change that, saving more babies and sparing families from heartbreak. But growing a child outside the body also challenges how we think about pregnancy and parenthood.
“This kind of device would create a new stage of human development, something we’ve never had to describe or regulate before,” says Elizabeth Chloe Romanis, a medical law scholar at Durham University.
Artificial wombs raise difficult questions that scientists, bioethicists, and legal experts are grappling with before human trials begin: How will this technology change the way we preserve life, or even how we define life itself?
When I first saw the prototype, it didn’t remind me of a womb—it looked more like an aquarium.
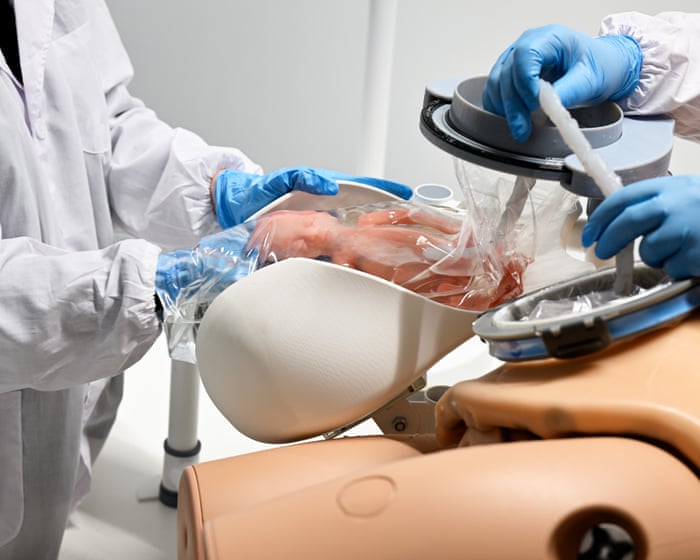
The glass tank sits on a waist-high platform in a bright lab in Aachen, Germany, part of the work by AquaWomb, a Dutch startup developing devices to help the smallest, sickest infants.
About the size of a home fish tank, the vessel sits under fluorescent lights so technicians can watch everything inside, though in practice it would be covered to mimic the darkness of the womb. Tubes run from the sides into filters that circulate synthetic amniotic fluid in a steady rhythm.
The design seems fitting for extremely premature infants, who often arrive looking as though they belong to another world—their skin translucent and delicate.With limbs as thin as matchsticks, these babies could float, drink, urinate, and grow inside the tank without ever touching air.
Myrthe van der Ven, a technical physician and CEO of AquaWomb, shows me how their prototype recreates the protected environment of pregnancy. The amniotic fluid is kept at 99.7°F, slightly warmer than a mother’s core temperature. A double-layered bag hangs in the center of the tank. The inner sac expands as the baby grows, starting at the size of a pomegranate at 23 weeks and reaching the size of an eggplant by 28 weeks. The outer silicone layer is firmer, flexible enough to withstand the baby’s kicks and help its muscles stretch and strengthen.
Van der Ven explains that the tank is the easy part—the real challenge lies with the lungs.
During a typical birth, a newborn’s first breath triggers the instinct to breathe, often marked by a cry that shows the lungs are working. But for extremely premature babies, this moment comes too soon. Their underdeveloped lungs can’t even manage a whisper, let alone supply enough oxygen to support the growing brain and body.
In neonatal intensive care units today, doctors intervene after birth, using ventilators and incubators to help these fragile organs function. But this mechanical support carries risks of lasting harm. Once the lungs are exposed to air, they’re permanently activated—like a fish that can’t be returned to water.
Artificial wombs aim to avoid this crisis entirely. In AquaWomb’s design, the baby is delivered by C-section into a fluid-filled pouch, moving seamlessly from mother to machine. Inside the transfer chamber, doctors reconnect the umbilical cord to an artificial placenta—a fist-sized device equipped with delicate catheters to remove carbon dioxide from the blood and sturdy cannulas to deliver oxygen and nutrients.
If successful, the placenta provides the time the baby’s lungs aren’t ready for. If it fails, the baby has just two minutes before oxygen deprivation could cause permanent brain damage. Throughout this process, the baby stays submerged in fluid, unaware it’s being born.
“It’s like juggling ten balls,” says Frans van de Vosse, a cardiovascular biomechanics professor at Eindhoven University of Technology who advises the project. “Only the balls are on fire, and dropping one is not an option.”
If perfected, an artificial womb could redefine the limits of survival. This may be why the few labs developing such technology are cautious about what to call it.
AquaWomb refers to its prototype as a “womb-like life support system,” avoiding the loaded term “artificial womb.” Meanwhile, the Children’s Hospital of Philadelphia (CHOP), thought to be closest to human trials, calls theirs a “biobag.” CHOP made headlines in 2017 when researchers kept fetal lambs alive for 28 days, demonstrating that an artificial womb could support blood circulation and organ development in a living animal.
The entire field operates under a shroud of secrecy. CHOP declined to comment for this story. (Vitara Biomedical, the company that licensed CHOP’s technology, has raised over $125 million, suggesting preparations for clinical trials.) Some researchers mentioned that CHOP scientists have agreed to collaborate but later backed out.
This caution reflects both the intense scrutiny around reproductive technology and the understanding that, as van der Ven says, “in science, there’s evolutionary and there’s revolutionary.” An artificial womb would be the latter.
Her team isn’t focused on being first at any cost. “We don’t need to be the first. We want to be the best,” she explains. For her, that means designing a system where parents can interact with their developing baby—a key priority.She believes other researchers have overlooked this aspect. One AquaWomb design has access ports so parents can touch their babies. Another includes a “uterus phone” that plays voices, music, or heartbeats into the fluid at the same muffled volume a fetus would hear in the womb.
These interactions—touching a tiny foot, speaking into the liquid, feeling movement inside the bag—could boost long-term health for premature infants. But the importance of bonding during pregnancy goes beyond survival rates.
Studies of families using IVF or surrogacy show that when pregnancy doesn’t follow the usual path, parents—especially mothers—may find it hard to feel like real caregivers. Very premature births can trigger similar emotions, partly because prematurity often stems from medical issues with the mother, not the baby.
“They might feel they haven’t fulfilled their duty to protect and carry their child,” says Romanis. She believes any ethical alternative to natural pregnancy must support parents’ emotional needs as well as the baby’s physical ones.
In short, seeing your baby floating in a tank or enclosed in a bag could change not just how you bond with them, but how you view yourself as a parent.
Three months after losing her son, Beth joined a support group that meets twice a month for parents who’ve lost a baby late in pregnancy or soon after birth, often due to extreme prematurity. They talk about what to do with unused baby gifts or how to handle questions from family and coworkers. Many have children who might have been ideal candidates for an artificial womb.
When Beth invited me, I imagined a lecture hall or hospital meeting room. Instead, we met in the basement of a Boston church, where Wendy, the therapist leading the group, set up a circle of folding chairs. People drifted in slowly.
Juliette van Haren works with research parts for a transfer device and artificial womb for premature babies. Photo: TU/e [Eindhoven University of Technology/Bart van Overbeeke]
Only one of the 17 attendees today was a man—he and his husband lost their daughter when their surrogate miscarried late in the pregnancy. Across from me sat a girl who looked too young to order a drink, let alone to have had and buried a baby. Her bleached blonde hair brushed against Joanne’s salt-and-pepper hair; Joanne, nearly 60, has been coming for three years, joining over a decade after losing her son. The group also includes an English professor, a stay-at-home mom, a police officer, and a pediatric nurse.
In this circle, it’s clear that pregnancy loss affects people unevenly, but grief touches everyone equally.
No one knows how long the group has been around. People hear about it through word of mouth. “We tried making a Facebook group once,” Beth told me, “but it got taken over by anti-vaxxers asking if we’d had the Covid vaccine.”
“And then my account got suspended because I told them to fuck off,” Joanne snorted. “When I lost my baby, I lost my patience for dealing with grown-ass babies too.”
Wendy put a hand on Joanne’s wrist and winked at me. “As you can see, we’re very open here. You can ask us about your baby bags.”
“Biobags,” I corrected through hiccup-like laughs, surprised by their casualness. I explained that the technology is still years away from hospitals and would likely only be offered to infants born at 22 or 23 weeks, who have few other options.
“I lost my baby at 22 weeks,” Joanne cut in. “Are you saying this could have saved him?”
“Maybe,” I admitted. “But not for sure. Hypothetically, if your doctors thought he was a candidate for an artificial womb, would you have…”
“Absolutely,” Joanne insisted. “All I wanted was to save him. If my body couldn’t do it, then maybe this womb thing could.”
The parents hunger for any scrap of information to nourish theirWhat if my baby could have survived at 21 weeks? How much would it have cost? Would I have been able to see my daughter, to hold her?
When I describe the prototypes, two women grimace, but the others lean in, asking for photos. They imagine their children in an artificial womb, floating peacefully in a dreamlike state.
These parents don’t know exactly what the technology might have offered, but they feel deeply what they lost without it. Most haven’t taken apart their babies’ cribs. The pediatric nurse thinks about changing jobs because being near newborns makes her want to cry. Beth often holds her stomach as she talks, as if cradling a child who isn’t there.
Each parent is haunted by thoughts of what else they could have done. Many believe that more intervention, more technology, might have saved their babies.
Wendy pauses. “Is more always better?” She remembers having her daughter by C-section at 24 weeks and pleading with doctors for intensive care. Despite the risky birth, they saved the baby in the operating room, manually pumping her chest to circulate blood to her brain. For four months in the NICU, her daughter was connected to tubes and given medications. The procedures were expensive and painful, but her underdeveloped lungs needed more time to grow.
Wendy needed more time too. “The outlook was grim, but when it’s your child, you keep hoping for a miracle. I couldn’t let her go.”
After 131 days, her daughter died from a collapsed lung. For months, Wendy was consumed by guilt, her grief flowing hot and salty down her cheeks. “I felt so selfish,” she says. “Even though I’ve moved past those feelings, I wish I’d given her a more peaceful, shorter ending.”
While she supports developing artificial wombs, Wendy wonders how doctors will get true informed consent from desperate parents. “Maybe in the future, every parent will have access to this technology,” she says. “How will they know if it’s the right choice—for the baby or for themselves?”
A week after my first visit to the support group, Beth tells me, “I can’t stop looking at the photos of the lambs in the bags. Probably shouldn’t do it right before lunch.”
The footage from CHOP’s animal trials disturbed her—not just the sight of slick pink bodies floating in fluid, but also how it eerily resembled a scene from The Matrix, where human babies are grown in industrial pods.
For decades, both scientists and science-fiction writers have been chasing the idea of artificial gestation. In 1958, a Swedish team attempted to sustain seven “pre-viable” human fetuses in what was essentially a pressure-cooker incubator with a blood oxygenator. Their experiment lasted only a few hours, and today’s ethics boards would shudder at such a crude attempt. Still, for a moment, a thought experiment became real.
Today’s artificial womb prototypes aren’t meant to replace natural pregnancy. Yet when CHOP published its research on biobags, it sparked widespread speculation about “complete ectogenesis,” where development from fertilization to birth happens entirely outside a woman’s body.
Researchers have tried to downplay these ideas, presenting their work as a major scientific advance but a small step for parenting.
The Perinatal Life Support system from startup AquaWomb focuses on safely transitioning the baby from the mother to a water-filled incubator. Here, a specially designed, realistic baby doll shows how this could work.
The concept of complete ectogenesis is still so distant that it’s not worth worrying about yet.”It’s worth discussing in the context of current work,” says one scientist developing artificial wombs, who requested anonymity to avoid conflict with his public relations team. “Premature infants die daily, and this technology could save many of them. Debating sci-fi scenarios misses the real point.”
Other experts argue these concerns deserve attention. “Every aspect of reproductive futures matters,” says Romanis. “Science fiction and reality often overlap when considering potential problems.”
She notes that even early versions of artificial wombs would force parents into heartbreaking decisions. This reminds me of Wendy’s question about how parents, desperate to save their child, could fully grasp the risks of using such experimental technology.
However, clinicians like Christoph Haller, a Toronto cardiovascular surgeon, view artificial wombs as providing continuity rather than disruption. His team is testing a prototype on fetal pigs. “These conversations with parents aren’t completely new,” he explains. “We already ask them to make impossible choices about treatments. With any innovation, we’ll continue being transparent about its capabilities and limitations.”
But unlike IV drips or ventilators, artificial wombs come with built-in fantasies and fears.
This became clearer when I revisited the grief support group a month later. Beth brought up the artificial womb images again. Joanne agreed, “I keep looking at them too. If that technology existed, I might not be here. I’d have done anything for more time.”
Across the circle, a younger woman disagreed strongly. “That’s the problem—you might not have a choice. They’d force your baby into it regardless.” Though she didn’t specify who “they” were, her suspicion was palpable. “People already judge you as selfish if you refuse any medical intervention. This would just be another pressure.”
A man who lost his daughter during a surrogacy spoke up: “I wonder if my husband and I would have used a surrogate if this existed. Maybe the ethical choice would be avoiding putting another person through pregnancy risks.”
The room echoed with conflicting worries—that the technology might come too late for some, or too soon for others. In this circle of folding chairs, debates about nature versus technology, care versus coercion, became intensely personal. Regardless of one’s stance, these questions carry profound weight.
During my last visit with Beth, she wore paint-splattered overalls in an empty room marked with tape. Holding a large wet brush, she worked on nursery walls that still smelled fresh.
“This is the final step,” she told me.
After her son’s death, Beth couldn’t enter the nursery she’d prepared. Joining the grief group, she followed Wendy’s advice to “take baby steps” in clearing the room (“no pun intended,” Beth added tiredly).
Her first attempt ended just steps inside when her legs gave way. “The room felt like a shrine to someone who barely lived,” she recalled. A week later, she tried again, leaving with two stuffed animals. She returned after two days.
Gradually, Beth dismantled the nursery, detaching herself from each item: tiny crocheted boots, an unfinished scrapbook containing maternity photos with her now-ex-husband, their hands gently pressed together.She rested her hand on her pregnant belly. She had hired someone else to take apart the crib. As we discuss artificial wombs, she continues painting over the nursery walls. The mural on the far wall—a hand-painted mountain landscape—is the last to be covered. “If this technology were available, would I be a bad mother for not using it?” she asks herself.
Maha supports this ‘natural’ infertility treatment. Is it a conservative strategy to restrict IVF?
For the scientists and doctors leading artificial womb research, Beth’s concerns highlight the risk of letting sci-fi dreams obscure the real, life-saving potential of these advances. Yet they also capture a fundamental uncertainty of parenthood: the fear of choosing wrongly between what is possible and what is right for your child.
If Beth’s son had been born two decades later, his life might have unfolded differently. In one possible future, he develops in an artificial womb that saves him. Beth fills a scrapbook with Polaroids of her baby, his tiny toes tucked into knitted booties.
In another version, her son is placed in an artificial womb. The doctors know his chances are slim but want to give him every opportunity. Beth watches her baby grow inside the tank, hoping for a miracle. But the odds remain against him, and the miracle never comes.
Would Beth find comfort in knowing she did all she could to save him? Or would she always question whether her decisions extended his life—and possibly his suffering—only to reach the same end?
These are the questions at the heart of the broader debate about how we should use artificial wombs once they become a reality. While research labs and FDA committees may set the rules, it is in the choices of parents like Beth that these dilemmas truly come to life.
“There are no simple answers, for me or for any parent,” Beth reflects. For now, she just keeps painting.
Frequently Asked Questions
Of course Here is a list of FAQs about a machine that can sustain a babys life outside the womb covering a range of questions from basic to advanced
Beginner Definition Questions
1 What is this machine and what does it do
This machine often called an artificial womb is a technological system designed to replicate the environment of a natural uterus It would provide oxygen nutrients and a protective fluid environment to support a babys development from a very premature stage to full term
2 How is this different from a regular incubator
A traditional incubator supports babies who are already born but need help with warmth and breathing An artificial womb aims to completely replace the biological functions of the uterus and placenta mimicking the entire gestational process for a much earlierstage fetus
3 Why was this technology developed
Its primary goal is to dramatically increase the survival chances and improve health outcomes for extremely premature babies
Benefits Applications
4 What are the biggest benefits of this technology
The main benefits are saving the lives of extremely premature infants reducing the severe longterm disabilities often associated with extreme prematurity and providing a new option for pregnancies with lifethreatening complications for the birth parent
5 Could this help with infertility
Potentially yes In the future it could offer a new path to parenthood for individuals or couples who are unable to carry a pregnancy including single men samesex male couples and people with uterine factor infertility
6 Would this make pregnancy safer
It could make gestation safer for the fetus in highrisk situations It might also eliminate the physical health risks and bodily changes associated with pregnancy for the person who would otherwise carry the baby
Ethical Societal Questions
7 Who gets to use this technology Will it be fair
This is a major societal challenge We will have to decide if access is based only on medical need or if its available for elective reasons Theres a risk it could become a luxury only the wealthy can afford creating a new social inequality
8 What are the legal and parental rights for a baby in an artificial womb
This technology forces us to redefine parent Is the legal mother the egg donor the person who commissioned the process or both